|
|
|||
|
(Back to Preceding Week; on to Next Week) |
|
|
|
WATER, WATER EVERYWHERE
Old topographical maps of what is now Hilton Pond Center show a long gully scarring the landscape--a ravine that continued to deepen after torrential summer thunderstorms that often strike the Piedmont. In about 1955, previous owners of the property asked the U.S. Department of Agriculture's Soil Conservation Service to survey and build a dam as part of a national effort to slow erosion, create farm ponds for livestock and irrigation, and provide water reservoirs to be used when fires broke out in rural areas. The service advised a packed-earth structure they designed; in those days SCS even took care of engineering and construction. The resulting dam--complete with overflow standpipe--eventually created what is now one-acre Hilton Pond.
All text & photos © Hilton Pond Center The SCS also built a much larger dam about a quarter-mile downstream; this structure formed a second four-acre body of water partly on the western border of the Center's property (above). In the absence of any other known name, we call that one "Nothilton Pond." Historically, Hilton Pond was spring-fed at its northeastern edge, but that source no longer functions; now the pond serves mainly as a catch basin, receiving direct rainfall but mostly depending on runoff from surrounding uplands to counteract evaporation and seepage. Under drought conditions, the water level can get quite low; at full pond the impoundment is about 11 feet at its deepest point. Although we've spent countless days surveying terrestrial components of Hilton Pond Center, we've actually done very little investigation of the pond itself. We've cataloged only four species of fish plying its waters; a few days of seining undoubtedly would reveal more. And most of the 11 amphibians on our local checklist are aquatic--as are several of the turtles and snakes--but other than these creatures we know little about the floral, faunal, or chemical makeup of Hilton Pond. It was for this reason we jumped at the chance to collaborate when John McGill of York Technical College's biology faculty and adjunct instructor George Casey asked if they could bring their EVT 111 class ("Introduction to Water & Wastewater Treatment Laboratory") to analyze various water sources at the Center. We go 'way back with John--he was one of our science students at Fort Mill High in the 1970s--and he's brought several biology classes to Hilton Pond for memorable field trips. None of those involved water analysis, however, so we gladly set aside mid-July as a time for the group to visit.
The crew--two instructors and four students--arrived at about 8:30 a.m. and, after an orientation to the Center, unloaded a van full of equipment onto the back deck of the old farmhouse (above). John said this was a great opportunity because most of the students' previous work had been conducted in a laboratory; the setting at Hilton Pond presented challenges and conditions they would encounter when doing realtime on-the-job field testing.
To test the hand-dug well, Andrew Wescott of York and Eddie Benfield from Rock Hill lowered an apparatus (above right) designed to collect water not at the surface but after it had been submerged. Among other things, a sample so collected allows analysis of dissolved oxygen, which--in itself--can be an indicator of whether the water is capable of supporting aquatic life. Dissolved oxygen might not be all that important in drinking water--although it does affect the taste--but it's vital in a pond.
Later, Eddie used this same collection apparatus to get a deep-water sample from Hilton Pond, tossing it onto the surface (under the watchful eyes of instructor George and fellow student Camille Harrison of Rock Hill, above), but letting it sink before pulling a cord that allowed the device to fill with bottom water.
Andrew took a different approach to collecting surface samples (above), pulling on hip waders and venturing forth into dark and murky Hilton Swamp--and other aquatic habitats--before holding the mouths of his collection bottles barely underwater.
When gathering multiple samples in the field, it's critically important not to mix up the bottles, so George and John frequently reminded their students to cross-check the inscriptions on the collection bottles with those on the data sheets. Katharyne Tedford (above) of Rock Hill, who already has 15 years experience as an analytical chemist, is going back to school to refresh her field and laboratory skills--including accurate data recording.
One indicator of the health of any outdoor water source--be it river, stream, or pond--is the presence of macroinvertebrates. These are myriad small animals we don't need a microscope to see and identify--although a 10x hand lens may come in handy, especially for older eyes. One might expect things such as flatworms, segmented worms, snails, and many kinds of insect larvae from dragonfly nymphs to hellgrammites (larval Dobsonflies). The students sampled for macroinverts in several ways: 1) After all macroinvertebrate and water samples were collected, students and instructors returned to the makeshift outdoor lab on the back deck and made ready to employ the analysis tools at their disposal. Using techniques outlined in their lab manual (below right) and in guides provided by instrument manufacturers, the students were very careful to do the same procedures exactly the same way each time. All the students were familiar with each of the portable instruments on the deck, but for the sake of time one person ran all samples for a particular parameter. Katharyne, for example, worked her way through all the samples to be evaluated for their pH--the concentration of hydrogen ions (i.e., relative acidity-alkalinity), with 7 being neutral pH; numbers below that are acidic, those above are basic. Far more accurate than a piece of litmus paper, her battery-powered meter (below left) could read pH to two decimal places. Standardizing for temperature and humidity, she found surface water from Hilton Pond had an essentially neutral pH of 7.01, The task of testing for copper and nitrite ions in the water samples fell mainly to Camille, who used a portable Hach DR-2400 spectrophotometer to find that Hilton Pond had 0.07 milligrams/Liter of copper, while the lower pond read .03 mg/L. Too much copper would not be a good thing for a pond because too much of it will diminish the number of macroinvertebrates. No nitrites were detected from either impoundment; this is a good sign that excess fertilizer run-off from neighboring farms is not entering Hilton Pond and that aquatic bacteria are not converting ammonia (from fish or livestock waste) into nitrites that are quite toxic to small fish.
Camille was not instructed to test for iron, but one of her fellow students returned to the Center after the field trip and picked up additional samples to run back at the York Tech lab. These showed Hilton Pond had an iron level of 0.452 mg/L, while Nothilton Pond was at 0.427 mg/L. Iron content is good to know because it is a limiting factor in the growth of most kinds of bacteria; minimal iron means minimal bacteria. Considering all the iron-rich red clay that lies just under the topsoil here at Hilton Pond Center, we were a bit surprised iron levels weren't higher in the pond itself. We were NOT surprised, however, to learn the nearly stagnant water in our swamp bottomland showed iron concentrations at a whopping 4.89 mg/L--almost ten times higher than that of either of the two ponds. The remaining field tests conducted by the students included analysis of dissolved oxygen and identification of macroinvertebrates. Despite dredging, dip-netting, and hand-grabbing of bottom muck, class members were unable to find any concentrations of macroinvertebrates. This didn't surprise us--we've looked before and noticed a dearth of these small creatures--but instructor George Casey was flabbergasted to learn this apparently unpolluted pond in a relatively natural environment was almost devoid of macroinverts. This was a mystery until Eddie and Andrew ran their dissolved oxygen tests on a Hach spectrophotometer. Placing each water sample in a small ampoule with a reagent, Andrew shook the solution, wiped the glass to remove contaminants (above right), and handed the container to Eddie for a reading.
The spectrophotometer results were spectacular--and quite revealing--as indicated by color differences in the vials. As expected, the slow-moving swamp water (almost colorless, above right) had almost no dissolved oxygen (DO)--a mere 1.5 mg/L that would be insufficient for most plant and animal growth. By comparison, the ideal DO for many freshwater fish is about 7-9 mg/L, while trout species may require 9-12 mg/L--a level maintained only by unpolluted cool-water mountain streams that cascade constantly over rocks and pick up oxygen from the atmosphere. Surface water from Hilton Pond had a DO reading of 6.5 mg/L (darker purple at left above)--marginal but probably acceptable to most pond fish common to the Carolinas. It was a whole 'nother story for readings from the pond's deeper water, where DO levels were just 3.3 mg/L--almost too low for catfish and carp. In George's judgment, oxygen at such low concentrations on the bottom of Hilton Pond explains why the students were unable to find macroinvertebrates; the tiny animals simply don't have enough oxygen to thrive. And to make maters worse, along the pond margin where DO is sufficient water temperatures may be too high for the inverts, plus terrestrial predators such as Raccoons and Great Blue Herons prowl shallow waters and consume whatever small animal life they can find.
The big question might be why the deepwater DO levels are so low, and we think we know the answer: There are essentially no oxygen-producing plants growing in Hilton Pond. We suspect this came about nearly two decades ago when someone stocked the pond with a dozen or so sterile Grass Carp, Ctenopharyngodon idella (file photo, above). These herbivorous, voracious non-native fish are often used to control aquatic plant growth in freshwater ponds and lakes. They patrol the bottom, using their mouths to grub up and eat whatever vegetation they can find. We've even seen them swim near a pond bank, clamp down on an emergent plant, and twist and roll until the plant is uprooted. We didn't completely comprehend this whole water quality scenario until after the York Tech students and instructors had packed up their gear and departed the Center, so we hope all of them get a chance to review this photo essay as a field trip follow-up. We appreciate their conscientious work and professionalism and hope John McGill and George Casey make the visit an annual event so we can look for changes--or stability--in the water that is everywhere in and around Hilton Pond. All text & photos © Hilton Pond Center POSTSCRIPT #1: The York Technical College's water analysis students also took samples at Hilton Pond Center to evaluate for coliform bacteria, whose presence indicates contamination from leaky septic tanks or other human or animal fecal sources. Such evaluations can be done in the field, but results may be more accurate--and less expensive--in a laboratory setting. Thus, John McGill and students took samples back to York Tech, where they inoculated petri dishes with water from Hilton Pond and Nothilton Pond, and from Hilton Swamp that lies between them. After several days John sent us photos of the results.
We were relieved to learn there was essentially no coliform growth in the plates from Hilton Pond. The swamp was a different matter, however; its samples showed very high levels of coliform bacteria (above)--probably due to less water, stagnation, and higher traffic from White-tailed Deer, Raccoons, and other wildlife. The dish above contained just one milliliter of swamp water added to the growth nutrient. What was surprising was the number of coliform bacteria in the lower pond--800 CFU’s (colony forming units) per 100 mL. The maximum recommended safe level for recreational use (e.g., swimming) is 200. Nonetheless, Lake Wylie on the Catawba River in York County SC routinely tests over 2,000 CFU’s. We should point out that water from both the old hand-dug well and the tap at Hilton Pond Center were "normal" in that they contained a few bacteria, but none of these were coliform. Both sources produce water that is completely potable and, we might add, quite palatable. All text & photos © Hilton Pond Center POSTSCRIPT #2: Hilton Pond Center for Piedmont Natural History is open by appointment for field trips and investigatory projects for groups, including school classes from kindergarten through graduate school. For information on scheduling a visit for your school or organization, please see Guided Field Trips.
Comments or questions about this week's installment?
Thanks to the following fine folks for recent gifts in support of Hilton Pond Center for Piedmont Natural History and/or Operation RubyThroat: The Hummingbird Project. Your tax-deductible contributions allow us to continue writing, photographing, and sharing "This Week at Hilton Pond." (Please see Support if you'd like to make a gift of your own.)
IMPORTANT NOTE: If you ever shop on-line, you may be interested in becoming a member of iGive, through which nearly 700 on-line stores from Barnes and Noble to Lands' End will donate a percentage of your purchase price in support of Hilton Pond Center and Operation RubyThroat. We've just learned that for every new member who signs up and makes an on-line purchase within 45 days iGive will donate an ADDITIONAL $5 to the Center. Please sign up by going to the iGive Web site. It's a painless and important way for YOU to support our work in conservation, education, and research. "This Week at Hilton Pond" is written & photographed You may wish to consult our Index of all nature topics covered since February 2000. You can also use our on-line Hilton Pond Search Engine at the bottom of this page. For a free, non-fattening, on-line subscription to |

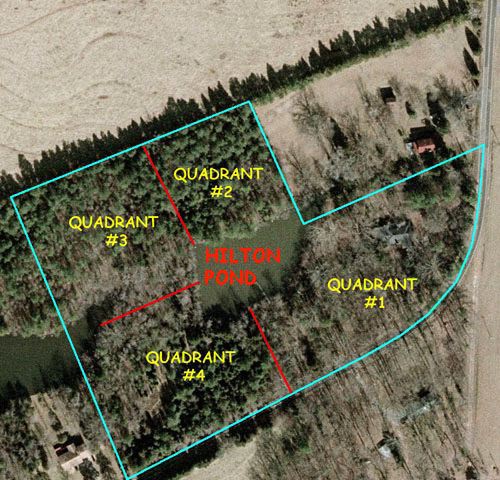

 George and John decided to have the students test several water sources available at the Center, those being: Hilton Pond itself, Nothilton Pond, and the bottomland (AKA "Hilton Swamp") between those two impoundments; the Center's tap water, which comes from a 160-foot deep well; and an old hand-dug rock-lined well that was the farmhouse's original water source when it was built back in 1918. Tap water sampling was easy--just turn on the faucet and hold out a collection bottle--but the other samples required either special equipment or more exertion, or both.
George and John decided to have the students test several water sources available at the Center, those being: Hilton Pond itself, Nothilton Pond, and the bottomland (AKA "Hilton Swamp") between those two impoundments; the Center's tap water, which comes from a 160-foot deep well; and an old hand-dug rock-lined well that was the farmhouse's original water source when it was built back in 1918. Tap water sampling was easy--just turn on the faucet and hold out a collection bottle--but the other samples required either special equipment or more exertion, or both.



 Simply squatting at the edge of the pond and grabbing a handful of bottom "muck" (a highly technical term for soil and decaying leaves); 2) tossing a rope tied to a dredge that was then dragged along the bottom to collect muck in a net (examined above by George and Camille); or, 3) fearlessly wading into the pond itself and sweeping the bottom with a small dip net (left, demonstrated by Eddie).
Simply squatting at the edge of the pond and grabbing a handful of bottom "muck" (a highly technical term for soil and decaying leaves); 2) tossing a rope tied to a dredge that was then dragged along the bottom to collect muck in a net (examined above by George and Camille); or, 3) fearlessly wading into the pond itself and sweeping the bottom with a small dip net (left, demonstrated by Eddie). Any chemical reagents were used in small quantities that were "reclaimed" after use, i.e., deposited into waste bottles that went back to the York Tech lab for proper disposal.
Any chemical reagents were used in small quantities that were "reclaimed" after use, i.e., deposited into waste bottles that went back to the York Tech lab for proper disposal. while Nothilton Pond was slightly acidic at 6.80--perhaps influenced by water that passes through Hilton Swamp (pH 6.62) before feeding into the lower impoundment. We were surprised the waters of Hilton Pond turned out to be neutral; metal parts on our dock rust so rapidly we thought for sure the results would show higher levels of acidity.
while Nothilton Pond was slightly acidic at 6.80--perhaps influenced by water that passes through Hilton Swamp (pH 6.62) before feeding into the lower impoundment. We were surprised the waters of Hilton Pond turned out to be neutral; metal parts on our dock rust so rapidly we thought for sure the results would show higher levels of acidity.
 Such a high level undoubtedly comes from the presence of iron-fixing bacteria (above)--slime-producing microorganisms that actually thrive on iron ions and concentrate them within their cells.
Such a high level undoubtedly comes from the presence of iron-fixing bacteria (above)--slime-producing microorganisms that actually thrive on iron ions and concentrate them within their cells.

 Thus, we suspect these fish have patrolled the bottom of Hilton Pond for the past 20 years, consuming virtually all green matter and effectively reducing the DO to a level not suitable for oxygen-loving macroinvertebrates. The good news is that only three or four of the original carp still survive; the bad news is that each of them is nearly four feet long and weighs 40 pounds. Grass Carp may indeed eliminate unwanted vegetation that foul the lines of a fisherman, but they remove plants that provide shelter for young fish and effectively eliminate DO necessary for the survival of big fish--and of their macroinvertebrate food. Unfortunately, it appears we're stuck with these last few carp until they eat themselves into oblivion.
Thus, we suspect these fish have patrolled the bottom of Hilton Pond for the past 20 years, consuming virtually all green matter and effectively reducing the DO to a level not suitable for oxygen-loving macroinvertebrates. The good news is that only three or four of the original carp still survive; the bad news is that each of them is nearly four feet long and weighs 40 pounds. Grass Carp may indeed eliminate unwanted vegetation that foul the lines of a fisherman, but they remove plants that provide shelter for young fish and effectively eliminate DO necessary for the survival of big fish--and of their macroinvertebrate food. Unfortunately, it appears we're stuck with these last few carp until they eat themselves into oblivion.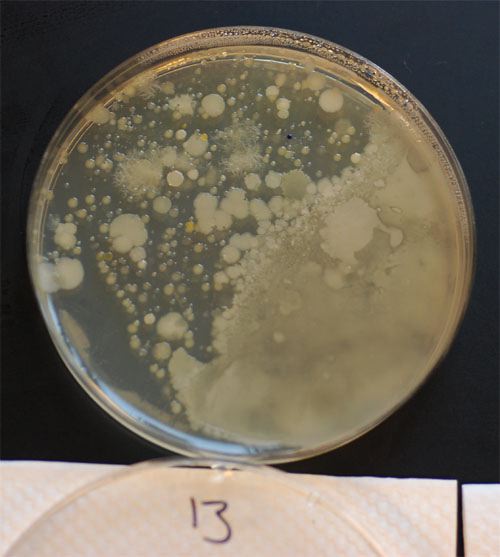


 Please report your
Please report your
